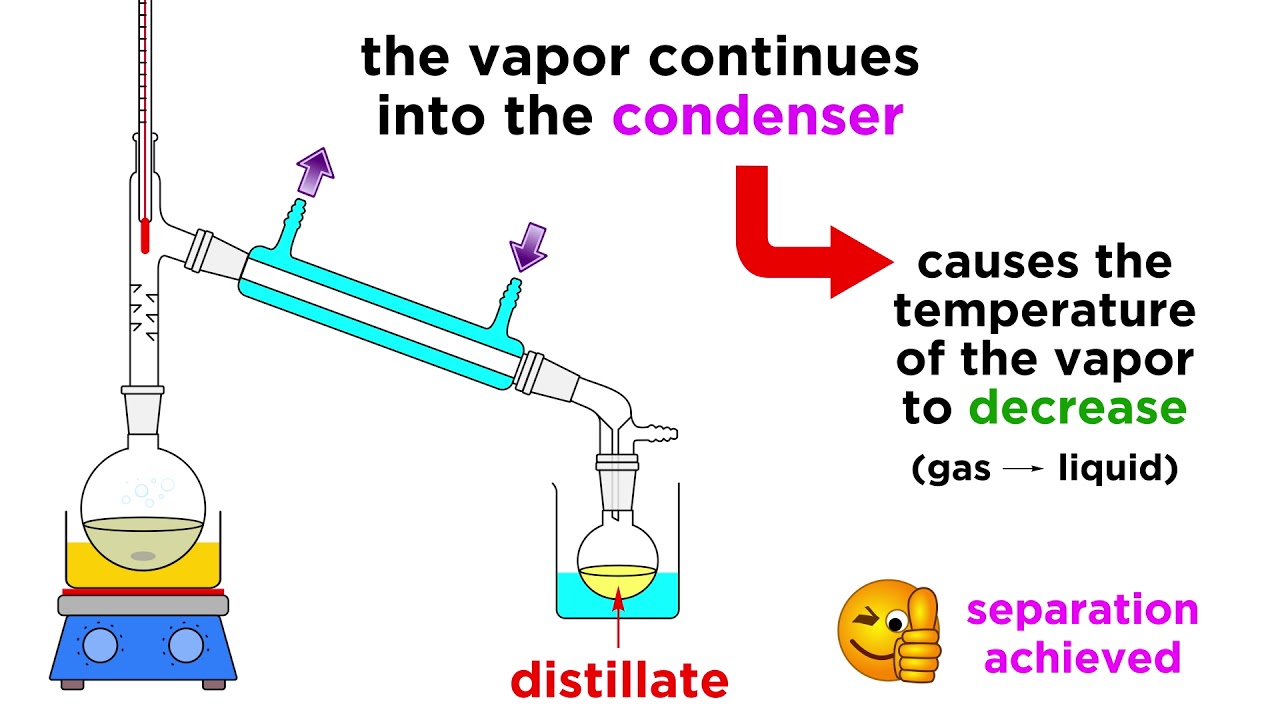
 We've got extraction and chromatography down, so let's learn one more separation technique. This one is pretty simple, it separates mixtures of liquids by differences in boiling point, and it's called distillation. If two liquids have very different boiling points, we should be able to heat the mixture up until one boils and the other doesn't, and we can collect the vapor somewhere else, and that's all there is to it. It's quite simple, in fact, but there are some important tips regarding the apparatus, so let's take a look!
We've got extraction and chromatography down, so let's learn one more separation technique. This one is pretty simple, it separates mixtures of liquids by differences in boiling point, and it's called distillation. If two liquids have very different boiling points, we should be able to heat the mixture up until one boils and the other doesn't, and we can collect the vapor somewhere else, and that's all there is to it. It's quite simple, in fact, but there are some important tips regarding the apparatus, so let's take a look! Subscribe:
ProfessorDaveExplains@gmail.com
General Chemistry Tutorials:
Organic Chemistry Tutorials:
Biochemistry Tutorials:
Classical Physics Tutorials:
Modern Physics Tutorials:
Mathematics Tutorials:
Biology Tutorials:
American History Tutorials:


0 Comments